Drug Development Documents

FDA Regulations
FDA Recall Audit Check Report Instructions
This file provides detailed instructions for completing the FDA Recall Audit Check Report. It includes information on recall details, program data, audit accounts, and consignee data. Useful for those involved in managing FDA recalls.

Clinical Trials
Clinical Trial Application Form Overview
This file provides a comprehensive clinical trial application form for requesting authorization from competent authorities. It includes critical sections for clinical trials on medicinal products for human use. Designed for sponsors, it guides users through necessary information for submission.

FDA Regulations
Forms FDA 3542a and 3542 Overview and Instructions
This file provides essential information regarding Forms FDA 3542a and 3542, including their purpose and instructions for use. It is vital for individuals submitting patent information to the FDA. Detailed guidance on how to fill out these forms is also included.

FDA Regulations
Traditional 510(k) Acceptance Checklist Instructions
This document provides detailed instructions for completing the Traditional 510(k) Acceptance Checklist. It includes preliminary questions and organizational elements necessary for FDA submissions. Users must follow the outlined guidance to ensure their submissions are administratively complete.

Clinical Trials
Clinical Trial Application Form CTA Guidance
This Clinical Trial Application Form (CTA) provides essential guidelines for applicants submitting their clinical trial protocols. It covers all necessary fields, including investigational product details and required documentation. Ensure compliance with the submission requirements to facilitate a smooth application process.
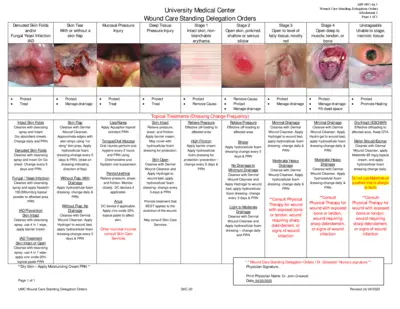
Clinical Trials
Wound Care Standing Delegation Orders Overview
This file provides comprehensive instructions and protocols for wound care management following standing delegation orders. It includes information about treatment for various skin conditions such as skin tears, fungal infections, and pressure injuries. Designed for healthcare professionals, this document serves as an essential guide for effective wound care.

FDA Regulations
Form FDA 3674 Certifications for Drug Applications
The FDA 3674 form provides essential certifications required for submitting drug, biological product, and device applications. This guidance helps sponsors, researchers, and investigations meet compliance standards. It's crucial for ensuring that necessary certifications accompany relevant applications.

Drug Testing
Drug Screen Point of Care Test Result Form
This document provides a comprehensive guide for drug testing at the point of care. It includes donor information, collector verification, and result interpretation. Suitable for use in various testing scenarios including pre-employment and random testing.

FDA Regulations
FDA Form 1572 Statement of Investigator Guidance
This document serves as a comprehensive guide for the Statement of Investigator (Form FDA 1572). It details the requirements for clinical investigators in research studies. Ideal for sponsors, clinical investigators, and IRBs seeking clarity on FDA guidelines.