Clinical Trials Documents

Clinical Trials
Clinical Trial Application Form Overview
This file provides a comprehensive clinical trial application form for requesting authorization from competent authorities. It includes critical sections for clinical trials on medicinal products for human use. Designed for sponsors, it guides users through necessary information for submission.

Clinical Trials
Clinical Trial Application Form CTA Guidance
This Clinical Trial Application Form (CTA) provides essential guidelines for applicants submitting their clinical trial protocols. It covers all necessary fields, including investigational product details and required documentation. Ensure compliance with the submission requirements to facilitate a smooth application process.
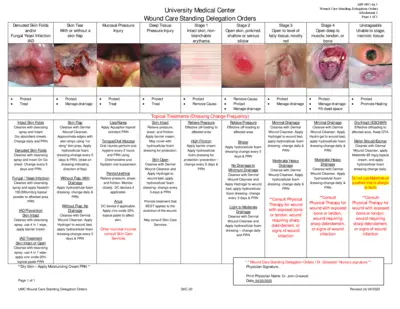
Clinical Trials
Wound Care Standing Delegation Orders Overview
This file provides comprehensive instructions and protocols for wound care management following standing delegation orders. It includes information about treatment for various skin conditions such as skin tears, fungal infections, and pressure injuries. Designed for healthcare professionals, this document serves as an essential guide for effective wound care.