Pharmaceuticals Documents

Pharmaceuticals
Assessment of Abuse Potential of Drugs Guidance for Industry
This document provides guidance for the assessment of abuse potential in drugs. It covers key decision points, recommended studies, and the process for NDA submission. This is crucial for ensuring drug safety and regulatory compliance.

Pharmaceuticals
Nurtec ODT Savings Program Terms & Conditions
This document provides detailed terms and conditions for the Nurtec ODT Savings Program. It includes eligibility criteria, instructions for pharmacists, and important disclaimers. Patients using the copay card should adhere to these guidelines to benefit from the program.

Pharmaceuticals
Abbreviations for Pharma Manufacturers
This file contains a list of manufacturers' abbreviations organized alphabetically, helping users to identify manufacturer names and their corresponding abbreviations.

Pharmaceuticals
Guidance for Out-of-Specification Test Results
This file provides industry guidance on investigating out-of-specification test results during pharmaceutical production. It includes detailed instructions and recommended practices for addressing OOS test cases. The document serves as a crucial resource for ensuring compliance with pharmaceutical quality standards.

Pharmaceuticals
Budesonide Draft Guidance on FDA Recommendations
This file contains the FDA's draft guidance on Budesonide including recommendations for studies to establish bioequivalence. It provides details about required in vitro and in vivo studies for different strengths of Budesonide. Important information about participant criteria for studies and the purpose of the guidance are also included.

Pharmaceuticals
Good Manufacturing Practice Inspection Application
This file contains the application for inspection of pharmaceutical manufacturing facilities. Users can request Good Manufacturing Practice (GMP) inspections. It is essential for compliance with national drug regulations in Uganda.

Pharmaceuticals
Membership Application Form for South Africa Pharmacists
This document is a membership application form for the Pharmaceutical Society of South Africa. It provides essential information for pharmacists seeking membership. Fill it out accurately to ensure proper processing.

Pharmaceuticals
Transfer of Ownership FDA Guidelines Manual
This document outlines the policies and procedures for the transfer of ownership of drug applications within the FDA. It serves as a guideline for applicants to ensure compliance with regulatory requirements during the ownership transition process. This manual aids in understanding responsibilities and the necessary steps for effective application management.
Pharmacy Services
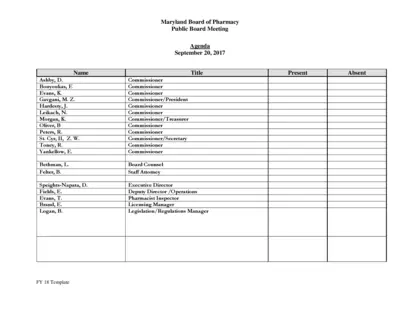
Maryland Board of Pharmacy Public Board Meeting Agenda
This file contains the agenda for the Maryland Board of Pharmacy's public board meeting on September 20, 2017. It includes reports from various committees and updates on operations, licensing, compliance, and more. The document is essential for stakeholders to keep track of board activities and decisions.

Pharmacy Services
California Intern Pharmacist Application Instructions
This document provides detailed instructions for applying for an Intern Pharmacist license in California. It covers processing time, required materials, and special cases for expedited review. Ensure all requirements are met to avoid application delays.

Pharmacy Services
Humana Pharmacy Contract Request Form
This form is used by pharmacies to request a contract with Humana. It includes sections for pharmacy details, types of services offered, and ownership information. Instructions for submission are also provided.
Pharmacy Services
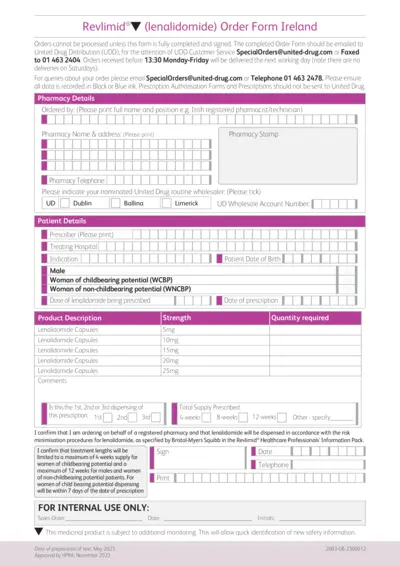
Revlimid Order Form Ireland Instructions
This document provides an order form for Revlimid® in Ireland. It includes essential details and instructions for healthcare professionals. Ensure to follow guidelines for proper completion and submission.