Pharmaceuticals Documents

Genetic Engineering
Room Reservation Form for College of Engineering
This is the official Room Reservation Form for the College of Engineering at the University of Arkansas. It is necessary for reserving rooms for events and requires careful completion of all sections. Ensure that you adhere to the guidelines outlined in the form for a smooth reservation process.

Drug Testing
Drug Screen Point of Care Test Result Form
This document provides a comprehensive guide for drug testing at the point of care. It includes donor information, collector verification, and result interpretation. Suitable for use in various testing scenarios including pre-employment and random testing.

Pharmaceutical Donations
Hair Donation Form for Children With Hair Loss
This file contains essential information regarding hair donations to Children With Hair Loss. It includes instructions for filling out the donation form, as well as details about the donation process. Donors can ensure their contributions are made effectively to support children with hair loss.
Genetic Engineering
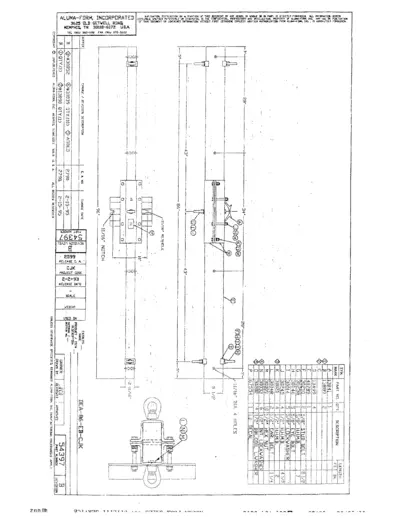
Aluma-Form Document Instruction and Components
This document provides detailed specifications for equipment related to Aluma-Form. It includes a list of parts along with their quantities and descriptions. Ideal for manufacturers and contractors needing essential information for their projects.

Genetic Engineering
IES ACES Mechanical Electrical Resident Engineer Form
This PDF document is an application form for Mechanical & Electrical Resident Engineers (RE) registration by the Institution of Engineers, Singapore. It contains detailed instructions for completing the application and important information regarding qualification verification. Ideal for engineers seeking accreditation to formally practice in Singapore.

Pharmaceutical Donations
Nike Donation Request Guidelines and Procedures
This document provides detailed guidelines for organizations seeking product donations from Nike. It outlines eligibility criteria, application procedures, and important policies. Organizations are encouraged to review the requirements carefully before submitting their requests.
Pharmaceutical Donations
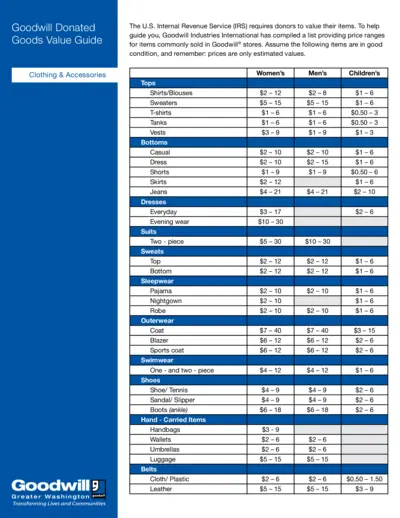
Goodwill Donated Goods Value Guide for IRS Compliance
This file provides a comprehensive value guide for donated goods to Goodwill. It helps donors estimate the value of their items for IRS reporting. Use it to determine fair market values for items typically sold in Goodwill stores.

Genetic Engineering
ASEAN Engineering Registry Application Guidelines
This document provides comprehensive guidelines and qualification requirements for the ASEAN Engineering Registry. It outlines how engineers can apply for registration and the necessary steps to complete the application process. The guidelines are essential for engineers seeking recognition and certification in the ASEAN region.

Genetic Engineering
IES ACES Application for RTO Registration Singapore
This PDF document provides essential information and instructions for applying for the Mechanical & Electrical Resident Technical Officer (RTO) registration in Singapore. It contains application fees, required documents, and processing times. Ideal for professionals seeking accreditation in engineering practices.

FDA Regulations
FDA Form 1572 Statement of Investigator Guidance
This document serves as a comprehensive guide for the Statement of Investigator (Form FDA 1572). It details the requirements for clinical investigators in research studies. Ideal for sponsors, clinical investigators, and IRBs seeking clarity on FDA guidelines.