FDA Form 3938 Drug Master File Instructions
This document provides essential guidelines for completing FDA Form 3938 for Drug Master Files (DMFs). It outlines the necessary steps for various stakeholders involved in the submission process. Use this resource to ensure compliance and streamline your application.
Edit, Download, and Sign the FDA Form 3938 Drug Master File Instructions
Form
eSign
Add Annotation
Share Form
How do I fill this out?
To fill out FDA Form 3938, begin by gathering required documentation to ensure accuracy. Carefully complete each section based on the instructions provided in this guide. Review the form before submission to avoid delays.
How to fill out the FDA Form 3938 Drug Master File Instructions?
1
Gather all necessary documents and information.
2
Complete each section of the form accurately.
3
Double-check for any errors or missing information.
4
Submit the form using the specified submission method.
5
Keep a copy of the submitted form for your records.
Who needs the FDA Form 3938 Drug Master File Instructions?
1
Pharmaceutical manufacturers need this file to submit Drug Master Files to the FDA.
2
Researchers require this document for compliance when producing new drugs.
3
Quality assurance teams use the form to provide necessary drug information.
4
Regulatory affairs professionals rely on this file for timely submissions.
5
Healthcare providers may need this form to ensure drug safety and efficacy.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the FDA Form 3938 Drug Master File Instructions along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your FDA Form 3938 Drug Master File Instructions online.
Editing PDFs on PrintFriendly is simple and user-friendly. You can easily modify text and images within the document as needed. After making your changes, download the edited PDF for your records.
Add your legally-binding signature.
Signing PDFs on PrintFriendly is a seamless process. You can add your signature directly to the document, ensuring that it is legally binding. Once you sign your PDF, download it for your files.
Share your form instantly.
Sharing PDFs on PrintFriendly is effortless and efficient. You can send the document via email or share it using a link. This feature helps you collaborate with colleagues and stakeholders with ease.
How do I edit the FDA Form 3938 Drug Master File Instructions online?
Editing PDFs on PrintFriendly is simple and user-friendly. You can easily modify text and images within the document as needed. After making your changes, download the edited PDF for your records.
1
Upload your PDF document to PrintFriendly.
2
Use the editing tools to make necessary changes.
3
Preview the document to ensure edits are correct.
4
Download the edited PDF to your device.
5
Share the document with others, if needed.

What are the instructions for submitting this form?
To submit FDA Form 3938, email your completed form and supporting documents to dmf.submissions@fda.gov or fax them to (301) 580-7176. Additionally, you can submit online via the FDA's Electronic Submission Gateway. For physical submissions, send your documents to: FDA, Office of Pharmaceutical Quality, 10001 New Hampshire Ave, Silver Spring, MD 20993. Ensure all documents are complete and accurate to avoid delays in processing.
What are the important dates for this form in 2024 and 2025?
The FDA Form 3938 has no specific deadlines; however, timely submission is recommended to ensure faster processing of your Drug Master File. Always check for relevant updates or changes to submission timelines. Stay informed about any upcoming regulations that may affect your submissions in 2024 and 2025.

What is the purpose of this form?
The purpose of FDA Form 3938 is to provide a structured means for submitting Drug Master Files to the FDA. It ensures that all necessary information is included for regulatory review and compliance. This form helps streamline the drug approval process, ultimately facilitating the availability of safe and effective medications.

Tell me about this form and its components and fields line-by-line.

- 1. Applicant Information: Details about the entity submitting the Drug Master File.
- 2. Drug Information: Specific details about the drug being submitted.
- 3. Manufacturing Process: Information on how the drug is manufactured.
- 4. Quality Control: Details about the quality assurance and testing methods.
- 5. Regulatory Compliance: Certification and compliance statements required.
What happens if I fail to submit this form?
Failure to submit FDA Form 3938 may result in delays in the review process. Incomplete or inaccurate submissions can lead to rejection, requiring resubmission. This can significantly hinder the drug approval timeline.
- Delays in Approval: Late submissions can lead to extended waiting times for drug approvals.
- Increased Costs: Delays may result in higher costs due to prolonged development phases.
- Regulatory Scrutiny: Errors can trigger further regulatory review, complicating the process.
How do I know when to use this form?

- 1. Initial DMF Submissions: Use this form for first-time Drug Master File submissions.
- 2. Updates to Existing DMFs: Submit this form when making amendments to your existing DMFs.
- 3. Compliance with FDA Regulations: Ensure your submissions comply with current FDA guidelines.
Frequently Asked Questions
What is FDA Form 3938?
FDA Form 3938 is used for Drug Master File submissions to the FDA.
Who should use this form?
Pharmaceutical manufacturers and researchers should use this form.
How can I edit this PDF?
You can easily edit the PDF using PrintFriendly's editing tools.
Is it possible to share the PDF?
Yes, you can share the PDF via email or through a link.
What documents do I need to complete this form?
You need supporting documentation related to the drug submission.
How do I submit this form?
Follow the instructions on the form for submission methods.
What happens if I make a mistake on the form?
Double-check your entries before submission to prevent issues.
Can I download the edited document?
Yes, you can download your edited PDF from PrintFriendly.
Is there a fee for using PrintFriendly?
PrintFriendly's tools for editing PDFs are free to use.
How do I reach customer support?
Contact our support team through the website for assistance.
Related Documents - FDA Form 3938 DMF

Requirement to Use Multiple Single-Sheet DEA Form 222s
This file provides guidance for DEA registrants on the requirement to use multiple single-sheet DEA Form 222s when transferring schedule I or II controlled substances upon the termination or transfer of a DEA registration or when discontinuing business altogether.

Customer Information Service Request for Demand Response
The Customer Information Service Request (CISR-DRP) form allows users to disclose their personal electricity-related information to third-party DRPs for obtaining Demand Response services under PG&E's Electric Rule 24. This document provides detailed instructions for completing the CISR-DRP form. Make sure to have a recent PG&E monthly Energy Statement before you start.

Detailed Instructional Document for Users
This document provides comprehensive guidelines on filling out the form. It includes important sections, required information, and steps for submission. Perfect for individuals and businesses looking to complete important documentation efficiently.

Liquor Licensee Rules and FAQs
This document provides essential information and answers frequently asked questions about liquor licensee regulations. It covers areas such as permissible sale hours, minor's presence at the bar, sale of drinks, happy hour rules, and more. Perfect for those working in licensed establishments in Pennsylvania.

Synergy Distributed Energy System Application Form Over 30kW
This file is used for applying to install or upgrade a renewable energy system and/or battery storage system with capacity over 30 kW up to 1MW, and transferring electricity into Western Power's network. It is required if ineligible for the Renewable Energy Buyback Scheme or Distributed Energy Buyback Scheme. Ensure you obtain a CS number from Western Power before filling out the form.

G99 Connection Procedures Guidance Document for WPD
This file outlines the connection procedures and requirements for Power Generating Modules per Engineering Recommendation G99. It details the steps and definitions needed for compliance. It also explains the roles of the customer, generator, and distribution network operator.

Net Energy Metering and Net Billing Tariff Application
This document is the application form for PG&E's Net Energy Metering (NEM/NEM2) and Net Billing Tariff (NBT) Interconnection for solar and/or wind electric generating facilities of 30 kW or less. It includes necessary customer and system information, interconnection types, system ownership details, contractor information, and description of generating facilities. Instructions also cover variances from Distribution Interconnection Handbook and Greenbook requirements.
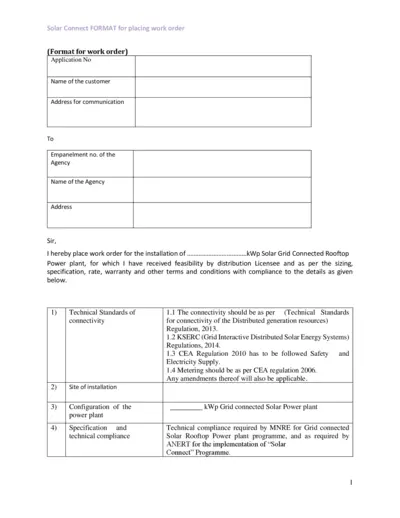
Solar Grid Connected Rooftop Power Plant Work Order
This file contains the format for placing a work order for the installation of a solar grid connected rooftop power plant. It includes technical standards, cost details, and warranty conditions. The document is essential for customers and agencies involved in the Solar Connect program.

National Fuel Gas Conversion Rebate Application Instructions
This file provides detailed instructions on applying for National Fuel's Natural Gas Conversion Rebate Program for 2024. It includes guidelines on the necessary documentation, eligibility criteria, and submission process. Ensure your application is complete and postmarked by March 31, 2025.
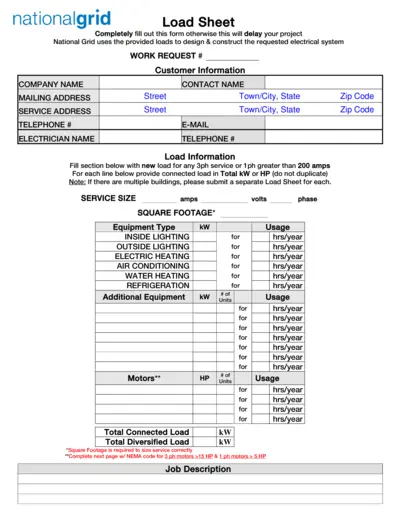
National Grid Electrical Load Sheet and Motor Data Form
This file contains the National Grid Load Sheet and Motor Data Form required for designing and constructing electrical systems. Users must completely fill out these forms to avoid project delays. It includes sections for customer information, load information, motor data, and welder data.

Demand Response Service Provider Registration Form
This file contains the Demand Response Service Provider Registration Application Form for California. It outlines the necessary information and instructions to apply for demand response services. Ideal for businesses involved in energy solutions and utilities.

Multimodal Dangerous Goods Form
This form is essential for shipping dangerous goods across multiple modes of transport. It adheres to international regulations ensuring safety during transport. Properly filling this form is crucial for compliance and effective logistics management.