DEA 222 Form Instructions for Schedule I & II Substances
This document provides detailed instructions for completing the DEA 222 Form, necessary for ordering Schedule I and II substances. It includes essential details for suppliers and purchasers to ensure compliance with DEA regulations. Follow the outlined steps to accurately fill out the form.
Edit, Download, and Sign the DEA 222 Form Instructions for Schedule I & II Substances
Form
eSign
Add Annotation
Share Form
How do I fill this out?
To fill out the DEA 222 Form, start by gathering the required supplier and purchaser information. Next, carefully input the order details, including the number of packages, package size, and name of the drug. Ensure all sections are completed accurately to avoid delays in processing your order.
How to fill out the DEA 222 Form Instructions for Schedule I & II Substances?
1
Gather necessary supplier and purchaser information.
2
Complete the order details with accurate drug information.
3
Ensure that no lines are skipped when filling out the form.
4
Sign and date the form appropriately.
5
Make a copy of the order form for your records.
Who needs the DEA 222 Form Instructions for Schedule I & II Substances?
1
Pharmacies need this form to order controlled substances legally.
2
Hospitals must use the DEA 222 Form to procure narcotic drugs.
3
Research laboratories require this form to obtain Schedule I drugs.
4
Veterinary clinics use it to order medications for animal care.
5
Manufacturers need it to supply registered entities with controlled substances.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the DEA 222 Form Instructions for Schedule I & II Substances along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your DEA 222 Form Instructions for Schedule I & II Substances online.
Edit your DEA 222 Form easily on PrintFriendly with our intuitive PDF editing tools. You can modify text, adjust fields, and ensure all entries are accurate before submission. Our platform makes it simple to create a compliant order form without hassle.
Add your legally-binding signature.
Signing the DEA 222 Form on PrintFriendly is straightforward. Utilize our digital signature feature to sign your form electronically. Once signed, you can download your updated form for your records.
Share your form instantly.
Sharing your completed DEA 222 Form is easy with PrintFriendly. You can share the PDF directly from our platform via email. Collaborate seamlessly with your colleagues by sharing your order form securely.
How do I edit the DEA 222 Form Instructions for Schedule I & II Substances online?
Edit your DEA 222 Form easily on PrintFriendly with our intuitive PDF editing tools. You can modify text, adjust fields, and ensure all entries are accurate before submission. Our platform makes it simple to create a compliant order form without hassle.
1
Open the DEA 222 Form in the PrintFriendly editor.
2
Click on the fields to edit supplier and order information.
3
Use the toolbar to adjust formatting if necessary.
4
Sign the form electronically using our signature feature.
5
Save your changes and download the updated PDF.

What are the instructions for submitting this form?
To submit the DEA 222 Form, send the completed document via fax to your designated DEA contact. For electronic submissions, ensure the form is properly signed and email it to the specified email address for your supplier. Additionally, you may also mail a physical copy to your supplier’s address as listed in the vendor information on your document.
What are the important dates for this form in 2024 and 2025?
The DEA 222 Form does not have specific deadlines but should be completed and submitted promptly to avoid delays in drug procurement. Ensure that you are aware of any state-specific regulations that may impact your submission. Regular updates may occur, so it’s advisable to check annually for changes in requirements.

What is the purpose of this form?
The purpose of the DEA 222 Form is to regulate the handling of controlled substances, specifically Schedules I and II. It ensures that both purchasers and suppliers maintain accurate records of these potent drugs, contributing to accountability and safety in dispensing. By filling out this form correctly, users help uphold the laws governing controlled substances.

Tell me about this form and its components and fields line-by-line.

- 1. Supplier Information: Includes the name and address of the supplier.
- 2. Order Details: Details such as number of packages, package size, and name of items.
- 3. Purchaser Information: Data about the purchaser including title and signature.
- 4. Alternate Supplier Identification: Details regarding any alternate suppliers if applicable.
What happens if I fail to submit this form?
If the DEA 222 Form is not submitted correctly, it may be returned for corrections, causing delays in orders. Improperly filled forms can lead to compliance issues with the DEA.
- Delays in Order Processing: Incorrect forms can result in significant delays in obtaining necessary substances.
- Legal Consequences: Filling errors may lead to legal repercussions for unauthorized or improper orders.
- Increased Scrutiny: Frequent errors can attract more attention from regulatory authorities.
How do I know when to use this form?

- 1. Ordering Controlled Substances: Utilize this form when ordering medications classified under Schedules I and II.
- 2. Record-Keeping Compliance: It is essential for maintaining compliance with DEA regulations.
- 3. Supplier Verification: Helps verify supplier information for drug distribution.
Frequently Asked Questions
How do I access the DEA 222 Form on PrintFriendly?
You can find the DEA 222 Form directly on our platform by searching for it in our documents section.
Can I fill out the DEA 222 Form online?
Yes, our platform allows you to fill out the DEA 222 Form online easily, making the process efficient.
Is the digital signature legally binding?
Our digital signatures comply with legal standards, ensuring they are recognized and binding.
What if I need to edit my form after saving it?
You can reopen your saved form in PrintFriendly at any time to make additional edits.
Can I share the DEA 222 Form with my team?
Absolutely, you can share the form via email directly from PrintFriendly.
Do I need an account to edit the PDF?
No, you can edit the DEA 222 Form without creating an account.
How can I ensure my information is secure on the platform?
We prioritize user experience and ensure all information is handled with care while using our PDF editing tools.
What if I make a mistake on the form?
You can easily correct any mistakes in the PrintFriendly editor before finalizing your form.
Can I print my completed form directly?
Yes, after editing, you can print your completed DEA 222 Form from our platform easily.
Is customer support available if I have questions?
Yes, our support team is here to assist you with any questions regarding the form.
Related Documents - DEA 222 Instructions

Requirement to Use Multiple Single-Sheet DEA Form 222s
This file provides guidance for DEA registrants on the requirement to use multiple single-sheet DEA Form 222s when transferring schedule I or II controlled substances upon the termination or transfer of a DEA registration or when discontinuing business altogether.

Customer Information Service Request for Demand Response
The Customer Information Service Request (CISR-DRP) form allows users to disclose their personal electricity-related information to third-party DRPs for obtaining Demand Response services under PG&E's Electric Rule 24. This document provides detailed instructions for completing the CISR-DRP form. Make sure to have a recent PG&E monthly Energy Statement before you start.

Detailed Instructional Document for Users
This document provides comprehensive guidelines on filling out the form. It includes important sections, required information, and steps for submission. Perfect for individuals and businesses looking to complete important documentation efficiently.

Liquor Licensee Rules and FAQs
This document provides essential information and answers frequently asked questions about liquor licensee regulations. It covers areas such as permissible sale hours, minor's presence at the bar, sale of drinks, happy hour rules, and more. Perfect for those working in licensed establishments in Pennsylvania.

Synergy Distributed Energy System Application Form Over 30kW
This file is used for applying to install or upgrade a renewable energy system and/or battery storage system with capacity over 30 kW up to 1MW, and transferring electricity into Western Power's network. It is required if ineligible for the Renewable Energy Buyback Scheme or Distributed Energy Buyback Scheme. Ensure you obtain a CS number from Western Power before filling out the form.

G99 Connection Procedures Guidance Document for WPD
This file outlines the connection procedures and requirements for Power Generating Modules per Engineering Recommendation G99. It details the steps and definitions needed for compliance. It also explains the roles of the customer, generator, and distribution network operator.

Net Energy Metering and Net Billing Tariff Application
This document is the application form for PG&E's Net Energy Metering (NEM/NEM2) and Net Billing Tariff (NBT) Interconnection for solar and/or wind electric generating facilities of 30 kW or less. It includes necessary customer and system information, interconnection types, system ownership details, contractor information, and description of generating facilities. Instructions also cover variances from Distribution Interconnection Handbook and Greenbook requirements.
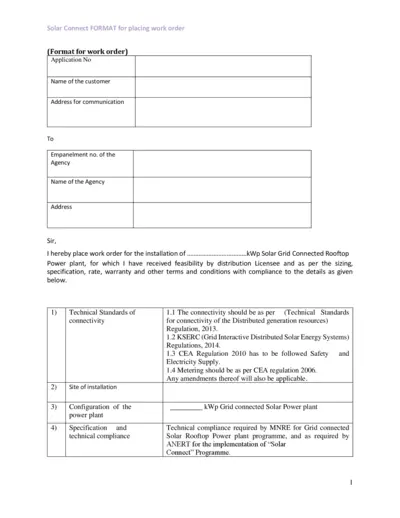
Solar Grid Connected Rooftop Power Plant Work Order
This file contains the format for placing a work order for the installation of a solar grid connected rooftop power plant. It includes technical standards, cost details, and warranty conditions. The document is essential for customers and agencies involved in the Solar Connect program.

National Fuel Gas Conversion Rebate Application Instructions
This file provides detailed instructions on applying for National Fuel's Natural Gas Conversion Rebate Program for 2024. It includes guidelines on the necessary documentation, eligibility criteria, and submission process. Ensure your application is complete and postmarked by March 31, 2025.
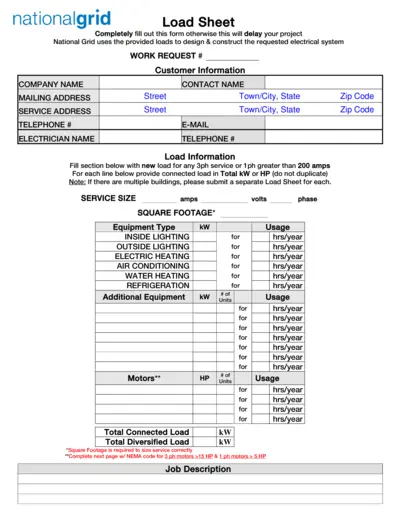
National Grid Electrical Load Sheet and Motor Data Form
This file contains the National Grid Load Sheet and Motor Data Form required for designing and constructing electrical systems. Users must completely fill out these forms to avoid project delays. It includes sections for customer information, load information, motor data, and welder data.

Demand Response Service Provider Registration Form
This file contains the Demand Response Service Provider Registration Application Form for California. It outlines the necessary information and instructions to apply for demand response services. Ideal for businesses involved in energy solutions and utilities.

Multimodal Dangerous Goods Form
This form is essential for shipping dangerous goods across multiple modes of transport. It adheres to international regulations ensuring safety during transport. Properly filling this form is crucial for compliance and effective logistics management.